Edwards’ EVOQUE Valve Replacement System First Transcatheter Therapy to Earn FDA Approval for Tricuspid Valve
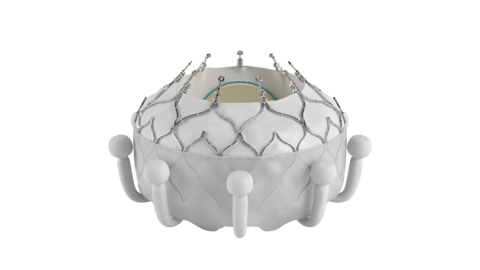

Edwards Lifesciences Corporation (NYSE: EW) today announced the company’s EVOQUE tricuspid valve replacement system is the first transcatheter therapy to receive U.S. Food and Drug Administration (FDA) approval for the treatment of tricuspid regurgitation (TR).
Edwards Lifesciences Corporation (NYSE: EW) today announced the company’s EVOQUE tricuspid valve replacement system is the first transcatheter therapy to receive U.S. Food and Drug Administration (FDA) approval for the treatment of tricuspid regurgitation (TR). The EVOQUE system is indicated for the improvement of health status in patients with symptomatic severe TR despite optimal medical therapy (OMT), for whom tricuspid valve replacement is deemed appropriate by a heart team.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240202805454/en/

(Photo: Edwards Lifesciences)
“Edwards has a long history of leading innovation and pioneering new therapies to address the unmet needs of patients with structural heart disease,” said Daveen Chopra, Edwards’ corporate vice president, transcatheter mitral and tricuspid therapies. “We are grateful for the strong collaboration with clinicians all over the world who contributed to the EVOQUE system now being available through FDA’s Breakthrough Pathway to provide a treatment option to the many patients in the US suffering with tricuspid valve disease.”
The EVOQUE system is comprised of a nitinol self-expanding frame, intra-annular sealing skirt and tissue leaflets made from the company’s proven bovine pericardial tissue. The EVOQUE valve will be available in three sizes, all delivered through the same low-profile transfemoral 28F system.
“Patients suffering with tricuspid regurgitation endure life-impairing symptoms and, until today, had no approved transcatheter treatment options,” said Susheel Kodali, MD, director, Structural Heart and Valve Center at Columbia University Irving Medical Center/New York-Presbyterian Hospital and TRISCEND II Study Principal Investigator. “The EVOQUE system is able to replace the native tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of patients. We see significant improvements in patients’ symptoms and quality-of-life, including not feeling short of breath and being able to care for themselves, which ranked highest on a patient preference survey conducted at baseline with TRISCEND II pivotal trial patients.”
Successful six-month results from the randomized controlled pivotal trial, TRISCEND II, were presented at TCT 2023 and reported favorable safety and effectiveness outcomes, demonstrating superiority to OMT alone and meeting all primary endpoints. Key findings in the trial included significant reduction or elimination of tricuspid regurgitation and significant and sustained quality of life improvement, while demonstrating a favorable balance between risk and benefit.
In addition to the six-month cohort, 318 of the total 392 randomized patients completed a 1-year visit. The results showed favorable trends in the device group compared to the control group in the primary composite endpoints, including all-cause mortality, tricuspid intervention, heart failure hospitalization, KCCQ, NYHA and 6MWD. Edwards expects to present the full cohort of 392 TRISCEND II pivotal trial patients at TCT 2024.
The EVOQUE system received CE Mark approval in October 2023, making it the world’s first transcatheter valve replacement therapy to receive regulatory approval to treat TR.
About Edwards Lifesciences
Edwards Lifesciences is the global leader of patient-focused innovations for structural heart disease and critical care monitoring. We are driven by a passion for patients, dedicated to improving and enhancing lives through partnerships with clinicians and stakeholders across the global healthcare landscape. For more information, visit Edwards.com and follow us on Facebook, Instagram, LinkedIn, X and YouTube.
This news release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, but are not limited to, statements made by Mr. Chopra and Dr. Kodali, and statements regarding expected product benefits, patient outcomes, objectives and expectations and other statements that are not historical facts. Forward-looking statements are based on estimates and assumptions made by management of the company and are believed to be reasonable, though they are inherently uncertain and difficult to predict. Our forward-looking statements speak only as of the date on which they are made, and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement. Investors are cautioned not to unduly rely on such forward-looking statements.
Forward-looking statements involve risks and uncertainties that could cause results to differ materially from those expressed or implied by the forward-looking statements based on a number of factors as detailed in the company’s filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2022, and its Quarterly Reports on Form 10-Q for the quarters ended March 31, June 30, and September 30, 2023. These filings, along with important safety information about our products, may be found at Edwards.com.
Edwards, Edwards Lifesciences, the stylized E logo, Edwards EVOQUE, EVOQUE, and TRISCEND are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240202805454/en/
